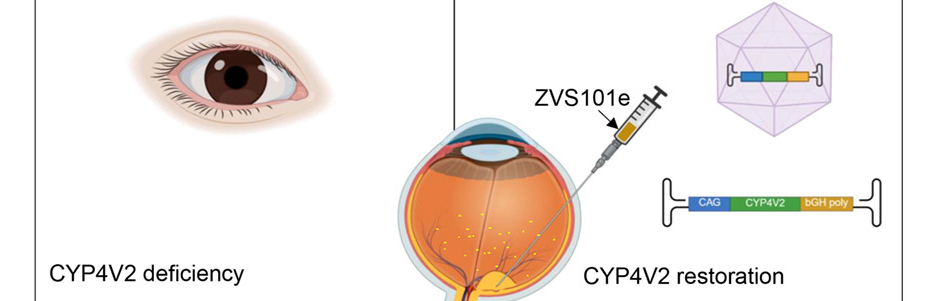
In a groundbreaking clinical trial, researchers confirmed that gene augmentation therapy can offer a potential cure for Bietti Crystalline Dystrophy (BCD), a rare inherited eye disease caused by changes in the CYP4V2 gene that normally helps the body process fats. The altered gene results in vision loss as crystals build up in the retina.
Findings from the human study, “Gene therapy for Bietti crystalline corneoretinal dystrophy: a phase 1/2 clinical trial,” were published in the peer-reviewed journal, Molecular Therapy.
Vinit Mahajan M.D., Ph.D., Stanford professor and vice chair of ophthalmology research, celebrated the results while expressing how rewarding it was to collaborate with Liping Yang M.D., Ph.D., a BCD expert from Peking University who led the successful phase 1/2 trial of the gene therapy ZVS101e.
Mahajan said, “Following the success of this trial, the team is in the planning stages of a phase-3 BCD gene therapy trial in the US, which brings new hope to the over 100,000 people affected worldwide.”
BCD is characterized by progressive atrophy of the retinal pigment epithelium (RPE), photoreceptors, and choriocapillaris, with many patients becoming legally blind by their 40s
In the phase 1/2 dose-escalation study, ZVS101e showed a favorable safety profile with no drug-related serious adverse events. The trial involved 11 East Asian participants who received subretinal injections of the viral vector at three ascending doses.
Results indicated the low-dose group (7.5×10¹⁰ vg/eye) achieved the most optimal efficacy, with an average best-corrected visual acuity (BCVA) improvement of 14.0 letters. Notably, 50% of these low-dose participants achieved clinically meaningful gains of at least 15 letters. Some patients—particularly in the medium and high-dose groups—experienced ocular inflammation, which was successfully managed with corticosteroids.
Mahajan said, “More is not always better. In this study, we learned that the lower dose may be safer and have better outcomes. This is why well-designed clinical studies are needed early on.”
Mahajan’s team previously used AI-generated models of the CYP4V2 protein to better understand how specific mutations damage protein function, helping determine which patients are best suited for clinical trials. Furthermore, powerful Adaptive Optics (AO) cameras designed by Stanford physicist Alf Dubra revealed new cyst-like structures in the retina that may be critical to understanding treatment response.
Research data is shifting how the medical community views BCD, as new findings suggest it is not just an eye disease.
Mahajan said. "Since CYP4V2 is active in several organs, including the liver and brain, BCD could be a broader metabolic condition,"
Study findings published in the American Journal of Surgical Pathology identified crystals in the liver of BCD patients, resembling those found in the eye and suggesting a striking manifestation of disordered fatty acid metabolism across the body.
By unravelling the mechanisms behind CYP4V2, scientists hope to pave the way for BCD treatments and other genetic eye diseases in the future. With a randomized, controlled phase 3 clinical trial in the planning stages, the transition from research to real-world treatment is closer than ever.