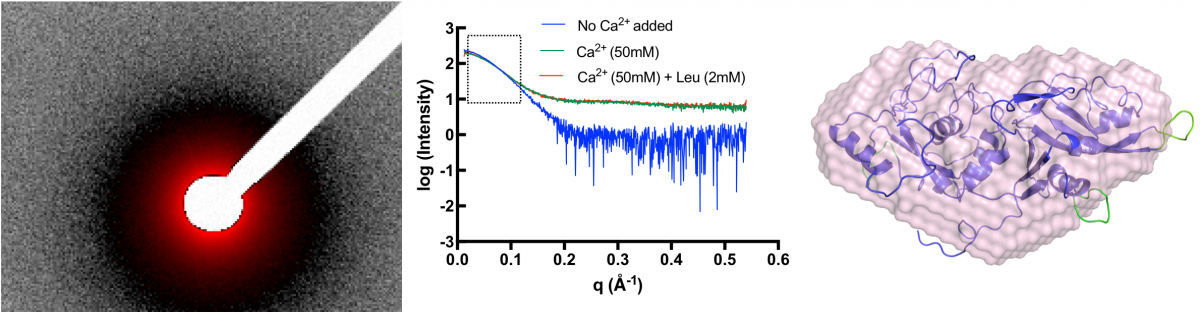
The structure of the CAPN5 protein has not been determined, making it difficult to design novel inhibitors to treat ADNIV, a rare eye disease caused by CAPN5 mutations. In the absence of a crystal structure, our lab has used small-angle x-ray scattering (SAXS) to determine the solution structure of CAPN5. SAXS-guided structural modeling suggests that CAPN5 adopts a more open conformation in the absence of calcium when compared to previously determined structures of other calpain proteins. These studies argue that pursuing higher resolution structural studies are necessary to understand the complex activity regulation prevalent in the calpain family and for the design of specific CAPN5 inhibitors.
20/20 Blog
Jul 26 2016